FDA warns some Claire’s cosmetics may contain asbestos

Source: (FOX 9) – The U.S. Food and Drug Administration is warning consumers not to use specific Claire’s cosmetic products because they may be contaminated with asbestos fibers.According to the FDA, the following products tested positive for tremolite asbestos during FDA testing:
• Claire’s Eye Shadows – Batch No/Lot No: 08/17
• Claire’s Compact Powder – Batch No/Lot No: 07/15
• Claire’s Contour Palette – Batch No/Lot No: 04/17
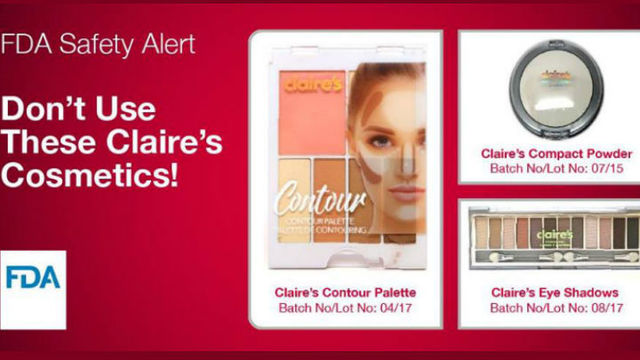
The Food and Drug Administration warns that come Claire’s makeup products tested positive for asbestos, a mineral linked with cancer. The regulator warned consumers not to use the products, although Claire’s disputed the findings.
The FDA said it issued the warning because Claire’s refused to comply with its request for a recall, and that the agency doesn’t have the power to force one.
Claire’s release a statement, noting that it removed the three products identified by the FDA from its stores “out of an abundance of caution,” and that it’s also removing any remaining talc-based cosmetics. But the company said the FDA’s test results mischaracterize fibers as asbestos. It said it tried to discuss the matter with the FDA, but the agency moved ahead with its warning.
The FDA conducted the tests after learning of reports of asbestos in products sold by retailers Claire’s and Justice in 2017. Talc is commonly found in makeup and personal care products, including baby powder and eyeshadow, and can be contaminated with asbestos, the FDA says.
